
Research in the Müller lab: Catalytic RNAs in the Origin of Life
ExpandThe Müller lab develops catalytic RNAs (ribozymes) to test how Life could have emerged from a prebiotic environment. This must have happened about 4 billion years ago (+/- 500 million years), in an environment lacking oxygen in the atmosphere. Since macromolecules from that time have degraded, if we want to find out how early life stages could have functioned we need to generate molecules in the lab that could have formed the first self-replicating and evolving systems.
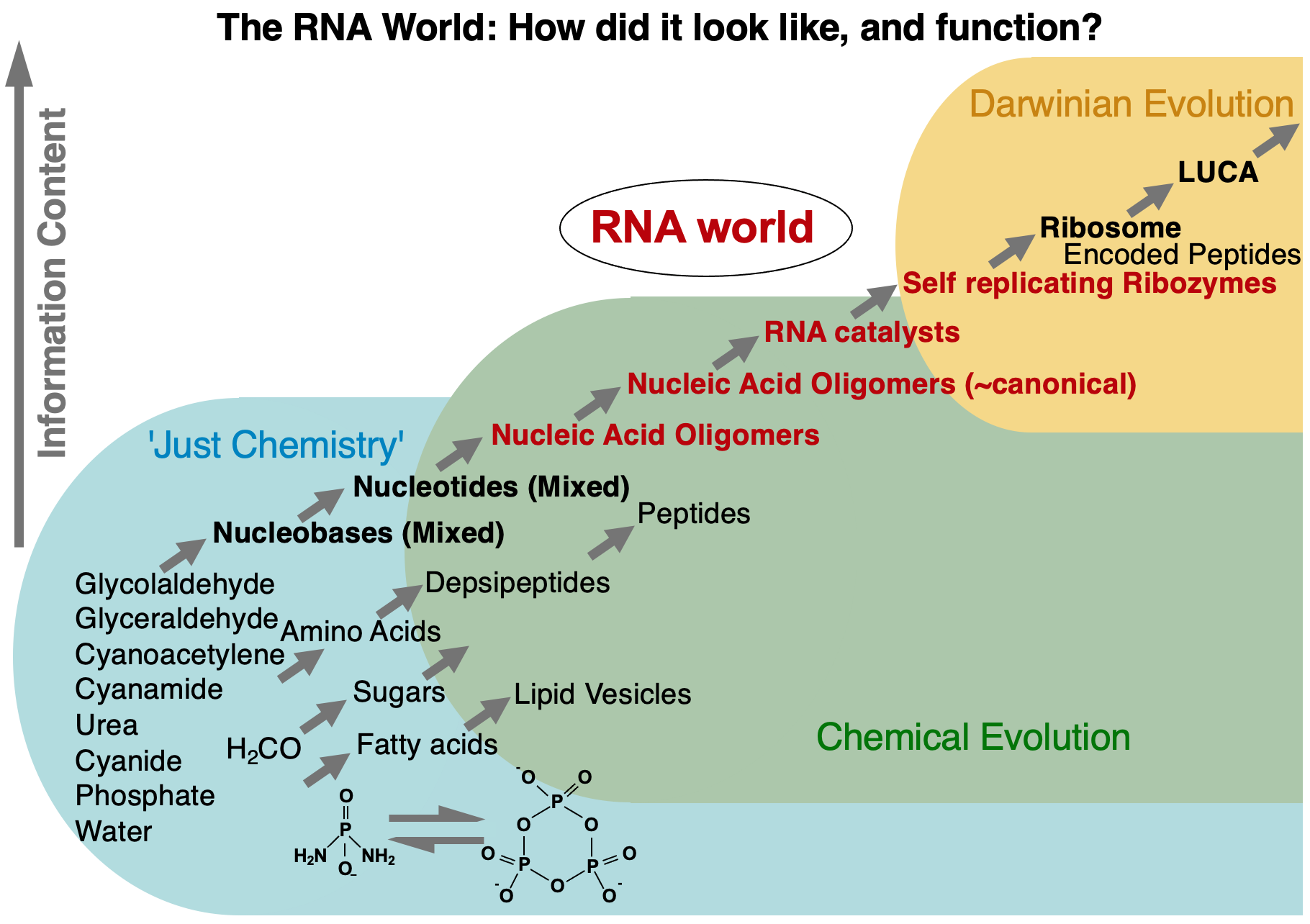
The origin of life must have proceeded in several stages, which allowed molecular building blocks to form, some kind of genetic information to persist, and self-replication to take hold to allow the process of Darwinian evolution to happen. The RNA world hypothesis states that RNA molecules - which are able to store genetic information like DNA and catalyze reaction slike proteins - could have formed self-replicating and evolving systems that preceeded today's DNA-RNA-protein organisms. Our research is focused on catalytic RNAs (ribozymes) as central actors in such early life stages.
A central requirement for any self-replicating system is a source of energy, and the Müller lab is focused on the prebiotically plausible molecule 'cyclic trimetaphosphate' (cTmp) as central energy source - similar to today's ATP in all biological organisms. We have used in vitro selection experiments to develop and optimize ribozymes that react cTmp with nucleosides and generated nucleoside triphosphates (NTPs), which would have been central in a self-repliating RNA system. We have performed selections for self-triphosphorylation ribozymes in the presence of different cofactors (peptides, lanthanides, oligomers) to test what kind of molecules in the environment could have helped the emergence of ribozymes. Our long-term goal is to build self-replicating systems of ribozymes that can show us how Life could have emerged from a prebiotic environment.
A Ribozyme that synthesizes GTP
Expand
The image shows the secondary structure of a GTP synthase ribozyme - a ribozyme that catalyzes the reaction between guanosine and the prebiotically plausible energy source 'cyclic Trimetaphosphate' (cTmp) to generate GTP. This ribozyme was selected in our lab using a novel emulsion-based selection system that identified sequences with GTP synthase activity from 2 x 1014 different sequences, dispersed in about 1016 emulsion droplets.
For an RNA world organism to replicate, chemically activated nucleotides are required. The Muller lab developed an emulsion-based selection technique to obtain ribozymes forming chemically activated nucleotides, specifically the formation of GTP from guanosine and the prebiotically plausible phosphorylation agent cyclic Trimetaphosphate (cTmp). This study is described in Akoopie et al (2021) Science Advances 7, eabj75487.
The ribozyme accelerates the rate of GTP synthesis by about 18,000-fold, which is a good rate enhancement for a ribozyme. However, it has a very low turnover rate, with each ribozyme generating only about two GTP molecules. This low turnover is likely limited by a slow product release, and was in part caused by the small size of the emulsion droplets used in the initial selection. We are currently improving the turnover by evolving the ribozyme in larger emulsion droplets, hoping for a turnover number of 100 or above.
The current ribozyme is specific for the synthesis of GTP and 6-thio GTP. We are currently developing variants of this ribozyme that generate ATP, CTP and UTP, with the goal of generating the full supply of NTPs for a self-replicating RNA system.
Prebiotic Peptide Analogs as cofactors for Ribozymes
Expand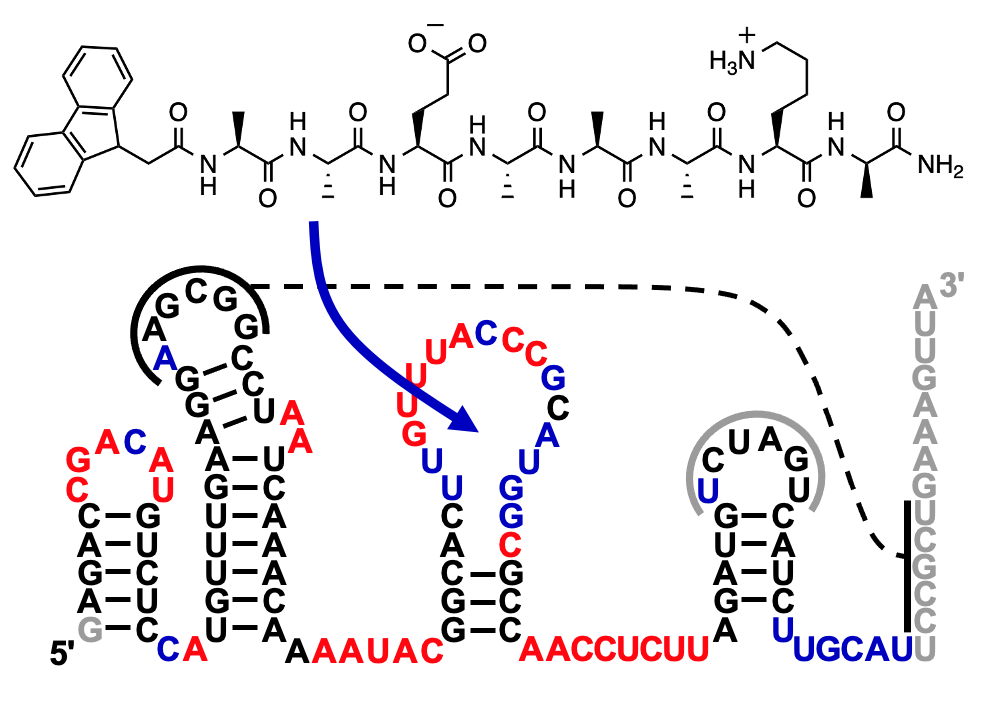
The image shows a peptide conjugate that was found to increase the activity of a ribozyme by ~900-fold. This polyaromatic hydrocarbon (PAH) at the N-terminus of the peptide is required for ribozyme activity. Instead of the PAH fluorene (shown), the PAH Naphthalene also mediates ribozyme function. Since PAHs including fluorene and naphthalene are abundant in the insoluble organic material of chondritic meteorites, it is possible that many early peptides existed as conjugates to PAHs and helped the emergence of ribozymes. The Muller lab is currently exploring different chemical modifications of peptides that may have aided the emergence of ribozymes in a prebiotic environment.
Assembly of Catalytic Oligonucleotide Complexes
Expand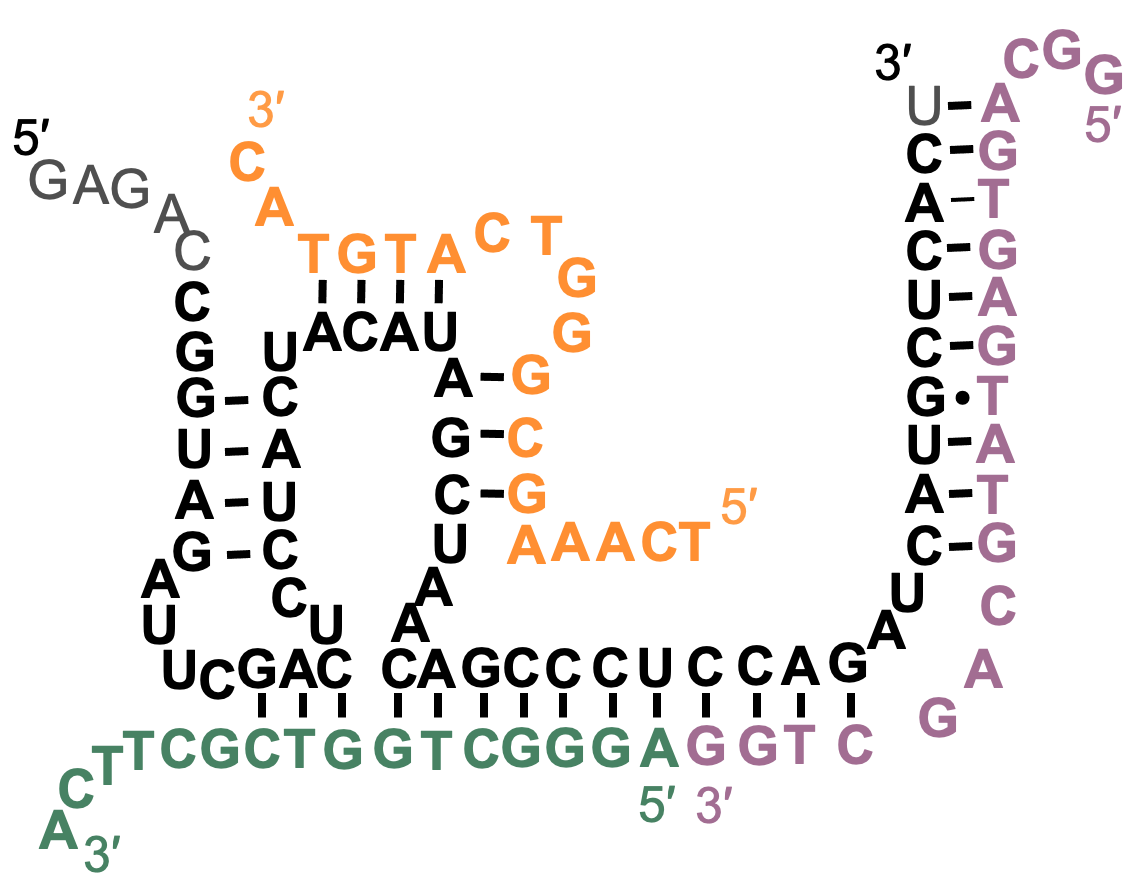
The image shows a catalytic complex of an RNA (black) with three different DNA oligomers (green, yellow, pink). This complex was obtained by the in vitro selection of a library of short RNA in the presence of a library of DNA 20mers, simulating a possible prebiotic scenario in which short oligonucleotides but not long polynucleotides may have existed. This study showed that catalytic complexes can assemble from combinatorial libraries of oligonucleotides, providing a novel view of how the first encoded catalysts could have looked like.
We are currently exploring the limits of this new principle - that libraries of short nucleic acid oligomers can form catalytically active complexes. This principle may get us closer to understanding how the first catalytic, self-replicating systems could have emerged in a prebiotic environments.
Trans-splicing group I intron ribozymes for therapy
ExpandGroup I introns are introns that do not need the spliceosome for their removal. Instead, theyr fold into a 3D structure that catalyzes two transphosphorylation reactions, which results in the excision of the intron and the joining of the flanking exons. These ribozymes have been re-designed to act in trans and recognize specific target sites on substrate RNAs, and replace the 3'-terminus with their own 3'-terminus. This can be used for therapeutic purposes, and is in clinical trials. One limiting factor for this approach is the low efficiency of trans-splicing. We have used our skills in the directed evolution of ribozymes to increase the trans-splicing efficiency (Olson and Muller 2012, Amini & Muller 2013, Olson et al 2014, reviewed in Muller 2017). The long-term goal is to reach an efficiency that is high enough for many therapeutic applications (Leier et al 2020).
In addition to 3'-replacement ribozymes we have re-designed a group I intron ribozyme to act in trans at two splice sites, remove internal substrate sequences of hundreds of nucleotides, and join the flanking exons that is then translated into functional proteins (Amini et al 2014). These ribozymes were named 'spliceozymes; because they are ribozymes that act like the spliceosome. We improved the efficiency of these spliceozymes by directed evolution in bacterial cells (Amini et al 2015). We continue to improve these spliceozymes through evolution experiments for therapeutic applications.